An international research team headed by Dr. Aaron LaForge from the research group of Prof. Nora Berrah in the Physics department at UConn has recently discovered a new type of decay mechanism leading to highly efficient double ionization in weakly-bound systems. The team has published its results in the science journal “Nature Physics”.
Ionization is a fundamental process where energetic photons or particles strip an electron from an atom or molecule. Normally, a much weaker process is double ionization, where two electrons are simultaneously emitted, since it requires higher-order interactions such as electron correlation. However, these new results show that double ionization doesn’t necessarily need to be a minor effect and can even be the primary ionization mechanism thereby getting two electrons for the price of one.
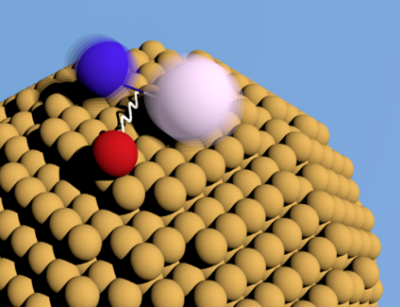
The enhancement is likely due to double ionization proceeding through a new type of energy transfer process termed double intermolecular Coulombic decay, or dICD, for short. The experiments were performed at the synchrotron, Elettra, in Trieste, Italy. There, electrons are accelerated to near the speed of light and then rapidly undulated through an alternating magnet field. In this way, the electrons emit short wavelength light which is needed to trigger dICD. The researchers produced superfluid helium droplets, which are cryogenic, nanometer-sized matrices capable of attaching various atomic and molecular species in order to perform precise spectroscopic measurements. In this case, dimers consisting of two alkali metal atoms were attached to the surface of helium droplets. The dICD process, schematically shown in Fig. 1, occurs through an electronically excited helium atom (red), produced by the synchrotron radiation, interacting with the neighboring alkali dimer (blue and white) resulting in energy transfer and double ionization. Although an alkali dimer attached to a helium nanodroplet is a model case, dICD is potentially relevant for any system where it is energetically allowed.
dICD belongs to a special class of decay mechanisms where energy is exchanged between neighboring atoms or molecules leading to enhanced ionization rates. Seemingly ubiquitous in weakly-bound, condensed phase systems such as van der Waals clusters or hydrogen-bonded networks like water, these processes can contribute to radiation damage of biological systems by producing particularly harmful low-energy electrons. dICD could strongly enhance such effects through the production of two low-energy electrons for each intermolecular decay.
Original publication:
A. C. LaForge, M. Shcherbinin, F. Stienkemeier, R. Richter, R. Moshammer, T. Pfeifer & M. Mudrich, “Highly efficient double ionization of mixed alkali dimers by intermolecular Coulombic decay”, Nature Physics (2019) DOI: 10.1038/s41567-018-0376-5